How to Set Dissolution Specifications
Dissolution testing is a crucial aspect of drug development and quality control. It determines the amount and rate at which active pharmaceutical ingredients (APIs) dissolve in a specific medium. The results of dissolution testing are used to evaluate the drug's bioavailability and ensure that it meets regulatory requirements.
Setting reliable and appropriate specifications for dissolution testing is essential for ensuring that the drug product is consistent in quality and performance. Failure to establish proper specifications can lead to incorrect dissolution profiles, which may lead to safety issues, reduced efficacy, and regulatory non-compliance.
Below are some points and best practices that need to be considered in setting specifications for Q, which is the percentage of dissolved active ingredient in the dosage unit.
Dissolution data of clinical trial material (CTM) batches
Gather dissolution data for all clinical trial material batches and plot them in one graph. This will make it easier to visualize the range of the data in terms of Q or the % of API dissolved at any given time point.
Dissolution data from stability testing
Gather dissolution data from stability testing experiments and plot them in one graph to get an idea of the variability of the data throughout the shelf life of the product.
Statistics - 3sigma
Perform statistics. Calculate 3 sigma or three times the standard deviation of the individual vessel raw data. This corresponds to 99.7% confidence level.
10% upper and lower bound for F2 similarity
Consider adding a 10% lower and upper bound from your CTM reference batch. This will ensure that the dissolution profiles of future drug product batches will be similar to the reference batch based on the F2 similarity calculations.
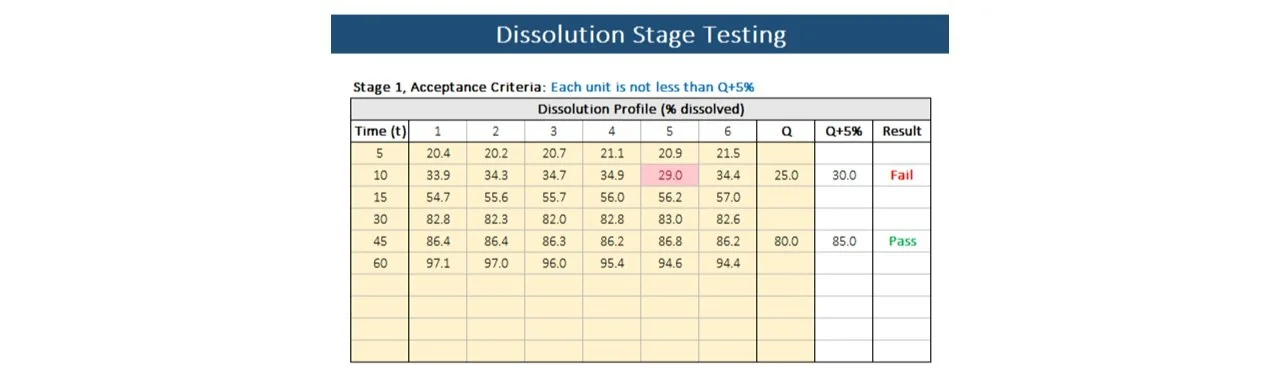
5. Dissolution stage testing
Consider the acceptance criteria for stage testing outlined in the table below and adjust Q accordingly.
Related Article: Dissolution Stage Testing in Pharmaceutical Analysis.
Related Article: How to Perform Dissolution Stage Testing According to the FDA Guidance.
Automate your stage testing calculation for dissolution using this template.
Do you have any questions or suggestions? Feel free to reach us by clicking here.