How to Perform Dissolution Stage Testing According to the FDA Guidance
Dissolution testing plays a vital role in the pharmaceutical industry, serving as a critical step in the development and quality control of dosage forms. This essential testing enables the evaluation of both the rate and extent of drug release from a formulated product, allowing for a thorough assessment of the product's bioavailability and therapeutic effectiveness. With its ability to provide valuable insights, dissolution testing ensures the highest standards of quality and efficacy are met in pharmaceutical formulations.
To comply with dissolution requirements, the quantity of active ingredient dissolved from the tested dosage units must align with the specifications provided in Acceptance Table 1 from the FDA guidance. It is imperative to conduct testing across all three stages unless the results conform at either S1 or S2.
Reference: USP <711> Dissolution
To streamline calculations for stage testing, we have developed a template that determines whether your data meets the FDA dissolution guidance. By inputting the dissolution data and the corresponding Q values, the template will indicate whether each stage has passed or failed. This tool simplifies the process and ensures adherence to regulatory standards.
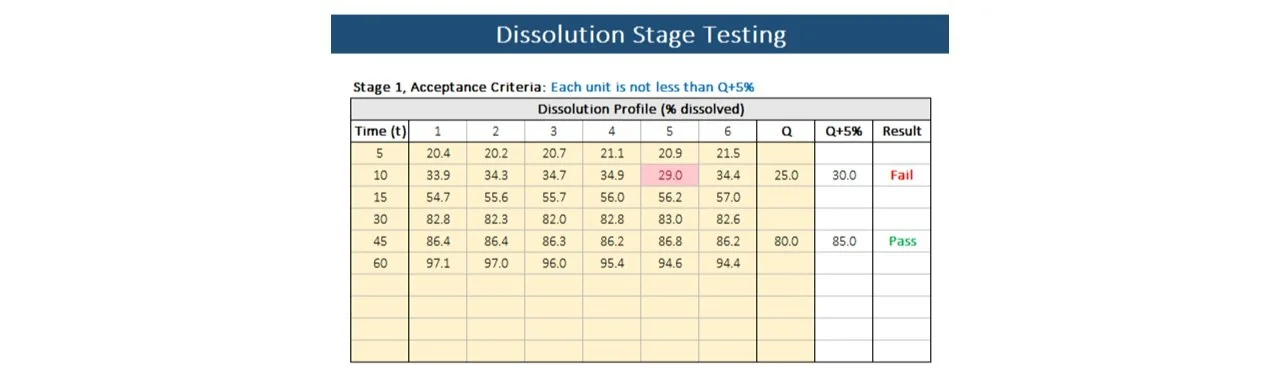
Stage 1
Input the dissolution time points in the "Time" column and the corresponding % dissolved values for each of the six individual dissolution vessels in columns labeled 1 through 6. In the "Q" column, enter the targeted amount of active substance, expressed as a percentage of the label claim, that should dissolve within a specified time. The template will automatically calculate Q+5% and display the result in the "Result" column, indicating whether the data passes or fails.
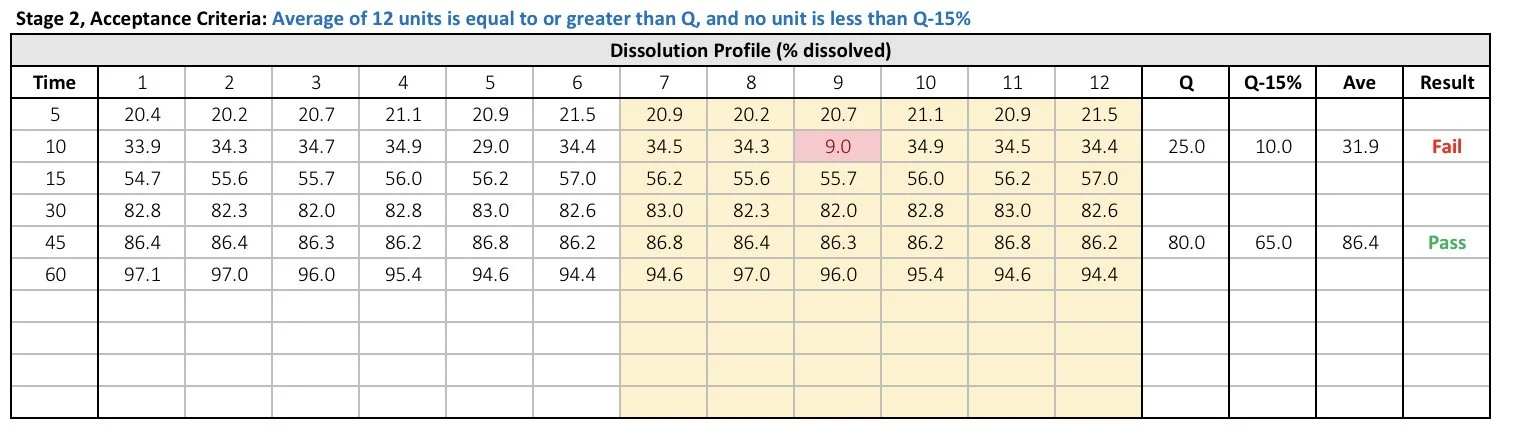
Stage 2
In the event that the generated data fails Stage 1, an additional six replicates must be performed. Record the new dissolution data in columns 7 through 12. The template will automatically copy the data from Stage 1 in columns 1 through 6. The template will also calculate Q-15% and the average. Furthermore, it will indicate whether the data has passed or failed the Stage 2 testing, as per FDA guidance.
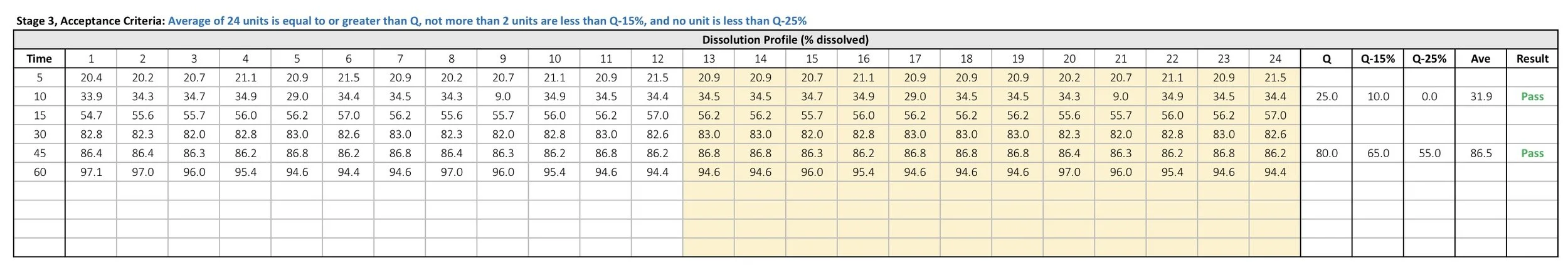
Stage 3
If the data continues to fail Stage 2 testing, additional 12 replicates must be performed. The template will automatically transfer data from both Stage 1 and Stage 2 testing to the new table. Enter the new dissolution data in columns 13 through 24. The template will calculate Q-15%, Q-25%, and the average. The template will indicate whether the data passed or failed Stage 3 testing according to the FDA guidance.
Feeling overwhelmed? Simplify the process with our dissolution stage testing template. Quickly perform these calculations and make your life easier!
Do you have any questions or suggestions? Feel free to reach us by clicking here.