Tablet Friability in Pharmaceutical Analysis
Friability refers to the tendency of a tablet to chip, crumble, or break during transportation. This can transpire when the tablet is being handled, packaged, or transported, potentially resulting in the administration of an incorrect dose to the patient. USP <1216> Tablet Friability shows the guidelines for determining the friability of compressed, uncoated tablets.
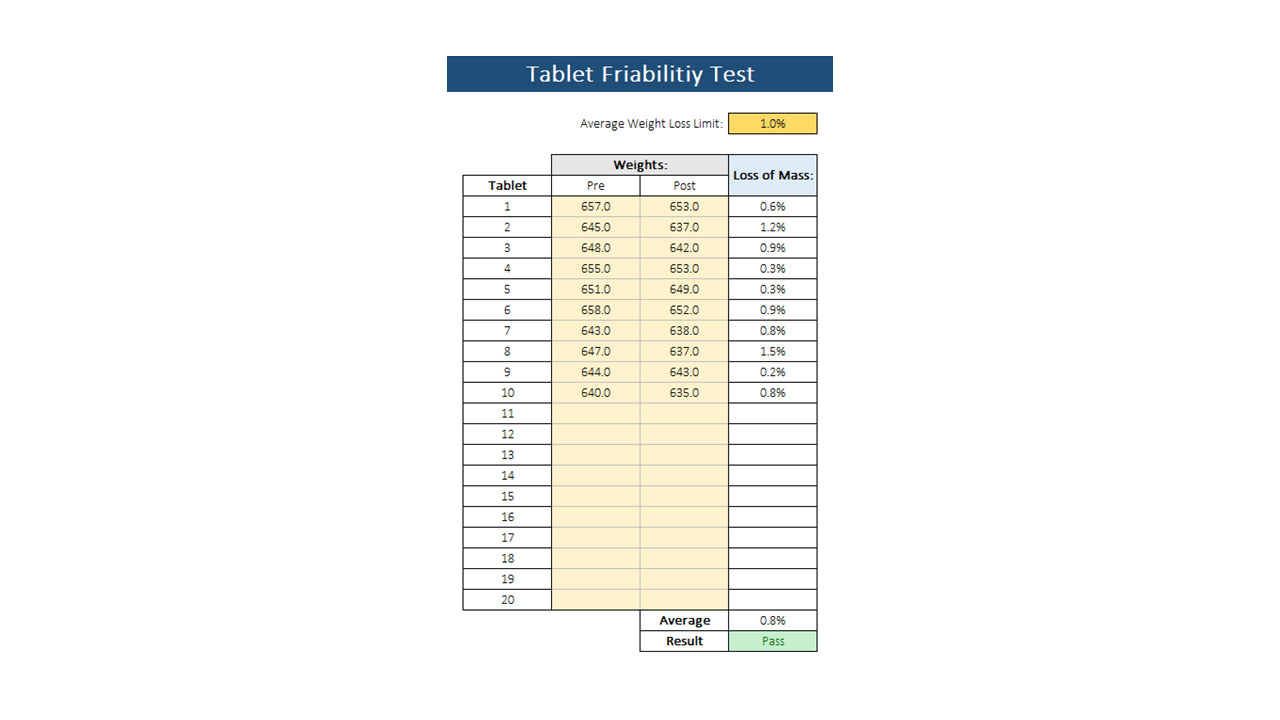
To test for friability, pre-weighed tablets must be placed in a tablet friability apparatus where the tablets are tumbled 100 times. After rotating, the tablets are weighed and the weight loss is calculated. If the results are difficult to interpret, it is recommended to repeat the test twice and get the mean of the three tests. A weight loss of 1.0% is considered acceptable for most products. If the tablets crack or break during the process, the samples fail the friability test.
Tablet Friability Calculator Preview
Do you have any questions or suggestions? Feel free to reach us by clicking here.