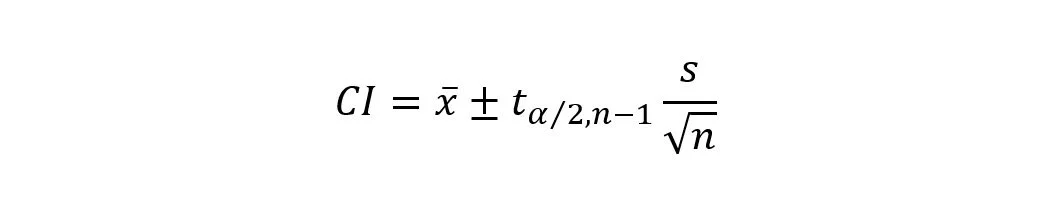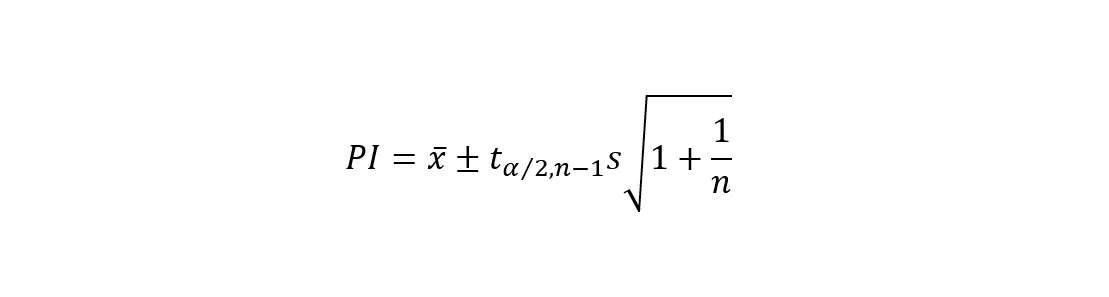Understanding Confidence Interval vs. Prediction Interval in Pharmaceutical Specification Setting
In the pharmaceutical industry, setting specifications for product quality is a critical aspect of ensuring safety, efficacy, and compliance with regulatory standards. Two statistical concepts, confidence intervals and prediction intervals, play distinct roles in understanding the variability of data and making informed decisions. This article explores the differences between these intervals and discusses their relevance in specification setting for pharmaceutical products.
Confidence Interval: Estimating Population Parameters
A confidence interval (CI) is a statistical range that provides an estimate of the plausible values for a population parameter, such as the mean or proportion. It is constructed based on sample data and is associated with a certain level of confidence, typically expressed as a percentage (e.g., 95% confidence interval).
Usage in Pharmaceuticals:
Confidence intervals are often employed to estimate the range within which the true population parameter is likely to fall.
They help quantify the uncertainty associated with sample estimates, providing a measure of the precision of those estimates.
Prediction Interval: Estimating Individual Future Values
A prediction interval (PI), on the other hand, goes a step further than confidence intervals. It not only estimates the range of plausible values for a population parameter but also considers the variability in individual future observations.
Usage in Pharmaceuticals:
Prediction intervals are valuable when predicting the range of possible values for individual future measurements.
They account for both the variability within the sample and the variability expected in future observations.
Choosing Between Confidence Interval and Prediction Interval in Specification Setting:
Type of Specification:
If setting specifications for the mean or proportion: Confidence intervals are more appropriate. They provide an estimate of where the true mean or proportion of the population is likely to lie.
If setting specifications for individual measurements: Prediction intervals are more suitable. They consider the variability of future individual observations and provide a range within which a future measurement is likely to fall.
Level of Certainty:
If a high level of certainty is required: Confidence intervals are preferable. They give a narrower range and are useful when a broad estimate is not acceptable.
If considering the potential range of individual values with more uncertainty: Prediction intervals are the choice. They provide a broader range, accounting for both current sample variability and future expected variability.
Risk Tolerance:
If minimizing risk is a priority: Confidence intervals may be favored. They offer a more conservative estimate of the range within which the true population parameter is expected to lie.
If accommodating higher uncertainty for individual measurements is acceptable: Prediction intervals might be more suitable.
Making Informed Decisions in Pharmaceutical Specification Setting
In the pharmaceutical industry, the choice between confidence intervals and prediction intervals for specification setting depends on the nature of the parameter being assessed and the level of certainty required. While confidence intervals provide estimates for population parameters, prediction intervals offer a broader perspective by considering individual variability in future measurements. A thoughtful consideration of the specific goals and risk tolerance is crucial to making informed decisions in pharmaceutical specification setting, ensuring both the safety of products and compliance with regulatory standards.